Visualizing Membrane Fusion
We use advanced imaging to see how proteins remodel membranes. Using viral entry as a model system, we map the complex machinery that drives fusion and cellular infection.
Biological function is driven by motion. We combine structural biology, biophysics, and AI to visualize molecular machines in action. By capturing the fleeting intermediate states of membrane fusion and assembly, we reveal new targets for therapeutic intervention.
We use advanced imaging to see how proteins remodel membranes. Using viral entry as a model system, we map the complex machinery that drives fusion and cellular infection.
Proteins are not static structures. We track the fleeting conformational changes—from prefusion to postfusion—to understand how molecular machines perform work.
We engineer novel antibody probes and use native-membrane environments (VLPs) to trap specific protein states, creating better platforms for structure-based drug design.
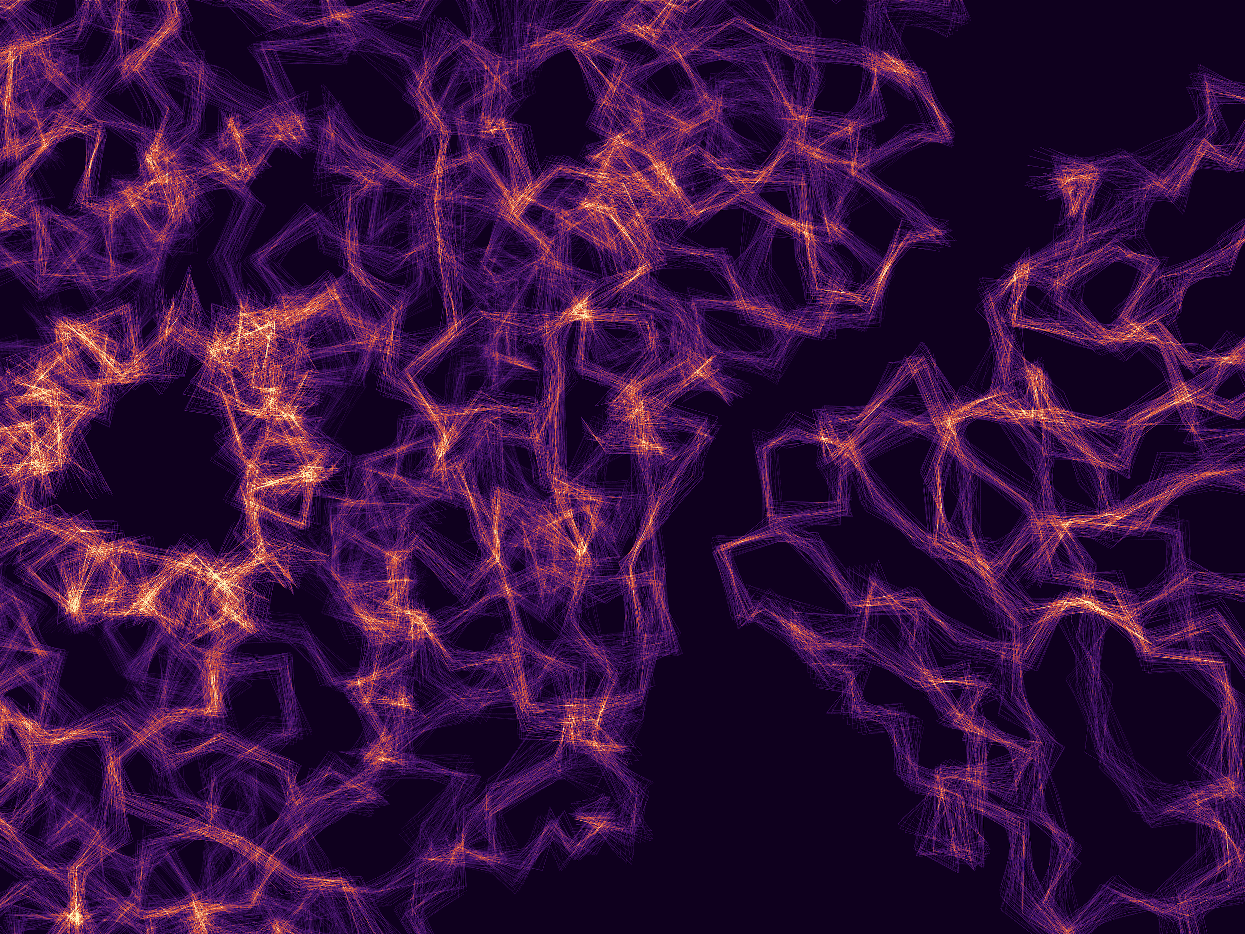
Using cryo-electron microscopy, we map the detailed structures of viral fusion complexes to understand how they merge viral and cellular membranes and how immune systems can block this process.
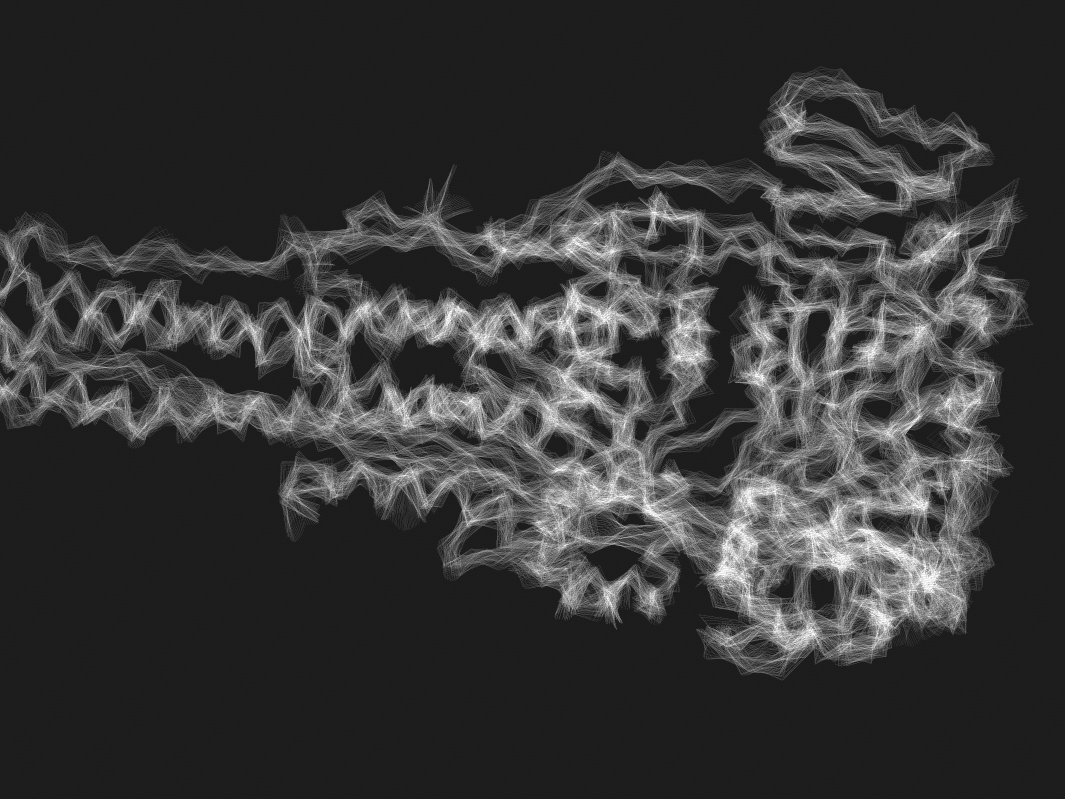
We are developing time-resolved techniques to freeze-frame protein dynamics during reaction, capturing the elusive intermediate steps that traditional methods miss.
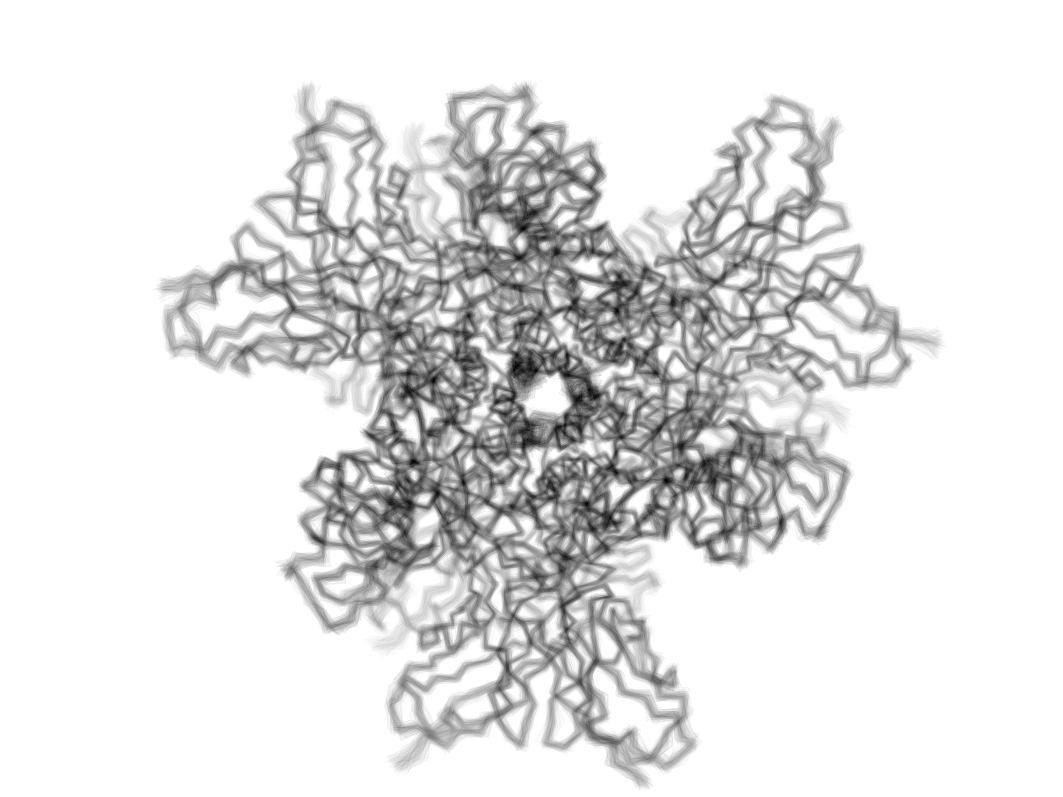
We leverage our understanding of viral conformational dynamics to engineer next-generation therapeutics. Using AI and structural insights, we design vaccines and antibodies that precisely target and arrest viral fusion.